
- #Free clinical trials trial
- #Free clinical trials iso
- #Free clinical trials professional
Some clinical trials involve healthy subjects with no pre-existing medical conditions. 9 Participant recruitment and participation.7.5 Aggregation of safety data during clinical development.6.1 Conflicts of interest and unfavorable studies.Only 10 percent of all drugs started in human clinical trials become approved drugs. Certain functions necessary to the trial, such as monitoring and lab work, may be managed by an outsourced partner, such as a contract research organization or a central laboratory. The sponsor may be a governmental organization or a pharmaceutical, biotechnology or medical device company. Clinical study design aims to ensure the scientific validity and reproducibility of the results.Ĭosts for clinical trials can range into the billions of dollars per approved drug. Clinical trials can vary in size and cost, and they can involve a single research center or multiple centers, in one country or in multiple countries.
#Free clinical trials trial
These authorities are responsible for vetting the risk/benefit ratio of the trial-their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.ĭepending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. Clinical trials generate data on dosage, safety and efficacy. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison.
#Free clinical trials professional
Our clinic regularly takes part in external quality control tests among professional laboratory testing and interlaboratory comparison trials.A clinical trial participant receives an injectionĬlinical trials are experiments or observations done in clinical research. The competence of the TomoClinic laboratory is also confirmed.
#Free clinical trials iso
TomoClinic has received international recognition and a valid quality certificate according to the requirements of the ISO 9001:2015 standard in the field of oncology, conducting radiotherapy and radiosurgical treatment services, as well as radiation diagnostics. Our oncological center has placement, equipment and supplies in the amount necessary and sufficient for conducting research, we also maintain the appropriate medical documentation. 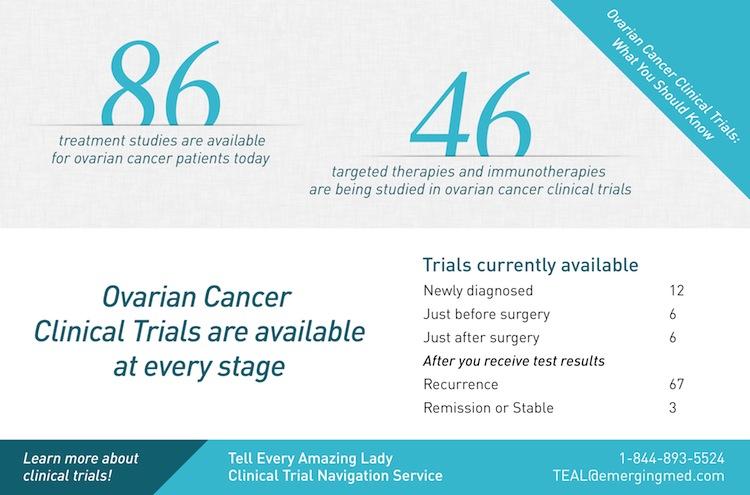 TomoClinic has conditions for storing medicines and the necessary base for the emergency medical care for the patients in case of complications during a clinical trial. We have successful experience in conducting clinical trials, we also know about the international requirements of good clinical practice and regulatory legal acts for conducting clinical trials in Ukraine. The medical staff has got sufficient training and experience in treating cancer patients. TomoClinic works in accordance with the Order of the Ministry of Health No. We meet all the criteria for a researcher and are ready to become one of the hospitals involved in clinical trials. In a second, third or fourth phase of research new combinations of already approved medicines may be studied. It is necessary to expand indications or register a new form of the medicine, as well as to obtain additional information about its safety. Next phase we need to study medicine after their approval. Participants receive the medicine during the third phase of the study, after this it became officially registered and released for sale. Treatment for 1000 unique patients with certain diseases is carried out with those medicines that have already proven their effectiveness all over the world and now must be tested in this country. Ukraine usually has clinical trials at third phase. Specialists should choose the treatment schedule, the dose of new medicine and know out about the risks. Testing is also carried out openly on a group of people with certain diseases (up to 300 participants). Second stage is the time to analyze the medicine effectiveness. These studies are “open”: both doctors and subjects have the information about medicine (dosages and treatment regimens). The safety of the medicine or proposed treatment should be analyzed. These are healthy volunteers or patients with certain medical conditions. The medicine is used for the first time during the first phase of the test and is performed on a small group (up to 80 participants).
TomoClinic has conditions for storing medicines and the necessary base for the emergency medical care for the patients in case of complications during a clinical trial. We have successful experience in conducting clinical trials, we also know about the international requirements of good clinical practice and regulatory legal acts for conducting clinical trials in Ukraine. The medical staff has got sufficient training and experience in treating cancer patients. TomoClinic works in accordance with the Order of the Ministry of Health No. We meet all the criteria for a researcher and are ready to become one of the hospitals involved in clinical trials. In a second, third or fourth phase of research new combinations of already approved medicines may be studied. It is necessary to expand indications or register a new form of the medicine, as well as to obtain additional information about its safety. Next phase we need to study medicine after their approval. Participants receive the medicine during the third phase of the study, after this it became officially registered and released for sale. Treatment for 1000 unique patients with certain diseases is carried out with those medicines that have already proven their effectiveness all over the world and now must be tested in this country. Ukraine usually has clinical trials at third phase. Specialists should choose the treatment schedule, the dose of new medicine and know out about the risks. Testing is also carried out openly on a group of people with certain diseases (up to 300 participants). Second stage is the time to analyze the medicine effectiveness. These studies are “open”: both doctors and subjects have the information about medicine (dosages and treatment regimens). The safety of the medicine or proposed treatment should be analyzed. These are healthy volunteers or patients with certain medical conditions. The medicine is used for the first time during the first phase of the test and is performed on a small group (up to 80 participants). 
It is a big process of testing a new medicine or method for cancer.






 0 kommentar(er)
0 kommentar(er)
